Which Element Has Similar Properties To Lithium?

Who Has The Highest Psr Rating Ever 2022, 2K MOBILE IS 10x BETTER THAN NBA 2K22! (NEW PARKS), 11.63 MB, 08:28, 130,534, COLETHEMAN, 2021-12-17T00:57:12.000000Z, 19, Yamaha PSR-E373 keyboard review – TrendRadars, www.trendradars.com, 590 x 410, png, , 5, who-has-the-highest-psr-rating-ever-2022, KAMPION
Its neighbor to the bottom (sodium) has similar properties to lithium. Group trends are very strong for the group 1 (alkali) and group 2 (alkaline earths) metals. Group 1 elements behave very similarly to each other. Here is an excerpt from one of my previous answers:
Li, na and k all ended up in the alkali metals group because of this pattern that grouped similar elements. They have similar chemical properties because they all have one valence electron which they lose easily to form a +1 ion. They all react vigorously with water to form hydrogen gas and. The second column on the periodic table of the chemical elements is collectively called the alkaline earth metal group: Beryllium, magnesium, calcium, strontium, barium, and radium. Because the outer electron structure in all of these elements is similar, they all have somewhat similar chemical and physical properties. Do lithium and sodium. A substance with a high activation energy is expected to have a _____ reaction rate. Write formulas for the following compounds, all of which contain polyatomic ions.
Which Element Has Similar Properties To Lithium Beryllium Explain ~ 46

Which Element Has Similar Properties To Lithium Beryllium Explain ~ 46

Which Element Has Similar Properties To Lithium Beryllium Explain ~ 46

Which element has similar properties to lithium Beryllium 2019 Explain

Which Element Has Similar Properties To Lithium Beryllium Explain ~ 46

Lithium melting and boiling point, MISHKANET.COM

Chemical properties of lithium and magnesium are similar because - NEETLab

The alkali metals - презентация онлайн
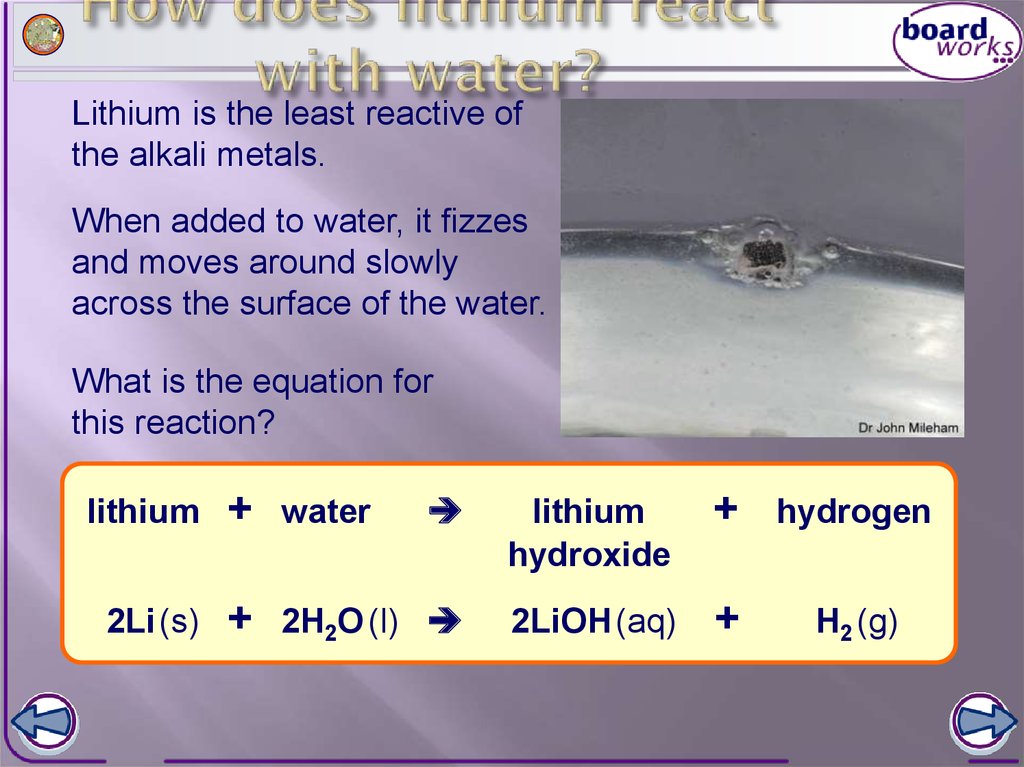
Lithium by Jared Glinn

8th Grade - Chapter 16 - Atomic Structure and Chemical Bonding

Komentar
Posting Komentar